OUR CLIENT
Lipari Foods is a wholesale food distributor and manufacturer that serves customers in 32 states across the U.S.. Lipari delivers a full range of grocery and retail goods sourced from over 40 global suppliers.
CHALLENGE
Manual documentation hindered Lipari operation and compliance
Lipari Foods, a grocery distributor and food manufacturer based out of Warren, MI, recognized a need to revamp and automate their FDA compliance processes due to three pressing problems:
- Improper DUNS number utilization: Unknown to Lipari, former importers were misleadingly using their DUNS number, thus exposing them to risk with the FDA
- FSVP documents managed manually: They used Microsoft OneNote to manually conduct the follow-up process for gaining updated FSVP documentation and stored the files in a folder on their hard drive
- Cost of sourcing suppliers: The Lipari sourcing team was flying around the globe looking for new FDA-compliant importers to provide alternatives for their supply chain

SOLUTION
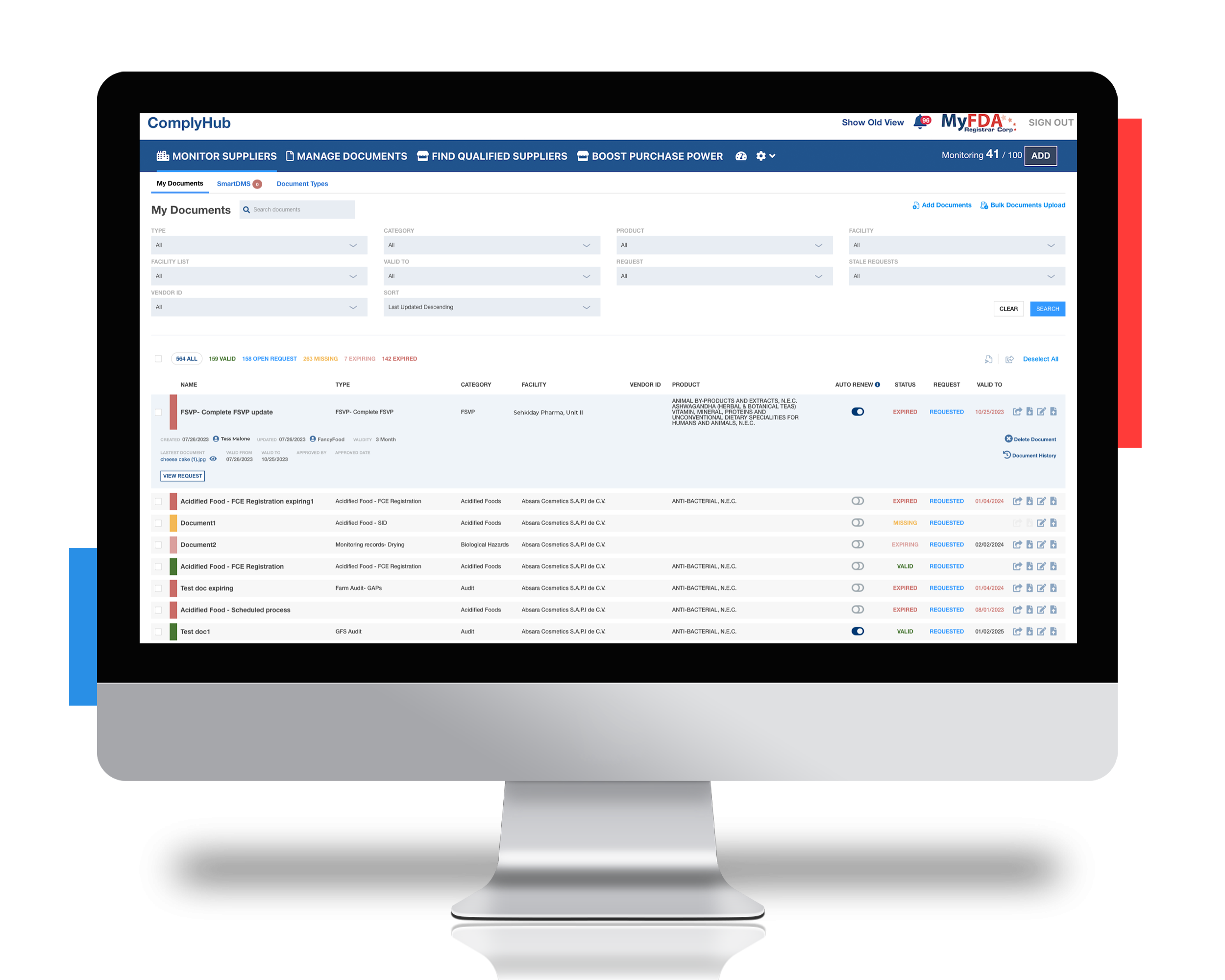
Working with Registrar Corp to update supplier list and manage FSVP documentation
With ComplyHub, Registrar Corp assisted Lipari to:
- Establish a process to remove invalid suppliers and prevent fraudulent activity of the former importers still using their DUNS numbers
- Organize all required FSVP documentation using the Manage Documents feature and receive alerts when updated files were needed from their suppliers
- Quickly locate FDA-compliant secondary or tertiary suppliers during supply chain issues with Find Qualified Suppliers’ active supplier directory

TESTIMONIAL
“It’s great having somebody that specializes in importation helping me not have to keep looking on the FDA website for import alerts.”
Justin Naramore, Director of Quality Assurance
IMPACT
SUPPLIERS MONITORED
40+
Suppliers’ FSVP compliance tracked successfully
TIME SAVED
2-3
Days per month freed up by viewing Import Alerts through Monitor Suppliers
COSTS AVOIDED
$50,000
Per shipment saved by importing from compliant vendors
Manage your business and
stay FSVP compliant 24/7

